Blood plasma and serum are important cornerstones in detecting and monitoring the progression of many diseases. As blood flows through every organ in the body, its protein composition uniquely reflects the body’s physiological state. In addition, blood samples are easy to collect, making plasma and serum attractive biospecimens to look for diagnostic biomarkers.
Traditionally, affinity-based assays have been used for protein biomarker discovery and validation in plasma and serum. However, these methods lack specificity and the ability to handle multi-analyte panels. Mass spectrometry (MS)-based proteomics solutions are, therefore, increasingly popular as they can simultaneously measure many hundreds of proteins and identify and quantify them more precisely than classical approaches. The combination of MS and stable isotope-labeled standard (SIS) peptides further increases the specificity, sensitivity, and quantifiability of the approach.
At Biognosys, we specialize in targeted proteomics services and have developed the most comprehensive and refined peptide kit for plasma proteomics available. Our PQ500™ Reference Peptides kit, an optimized set of 804 SIS peptides targeting 582 proteins, offers an array of benefits for plasma proteomics studies.
One of them is a greatly improved comparability across analytical platforms, making it especially helpful in large studies and cohort sizes. To showcase this benefit, we systematically assessed the comparability between several different LC-MS systems using a targeted proteomics workflow with PQ500™.
High Repeatability Between Instruments
When comparing plasma and serum from the same blood draw we found different characteristics between the two sample types, as was to be expected. Looking at the results from a single instrument (Figure 1A), levels of fibrinogens, clotting factors, and certain calcium-binding proteins were significantly different between serum and plasma. A statistically comparable pattern was observed when comparing multiple instruments (Figure 1B), indicating high repeatability of the workflow between different analytical platforms.
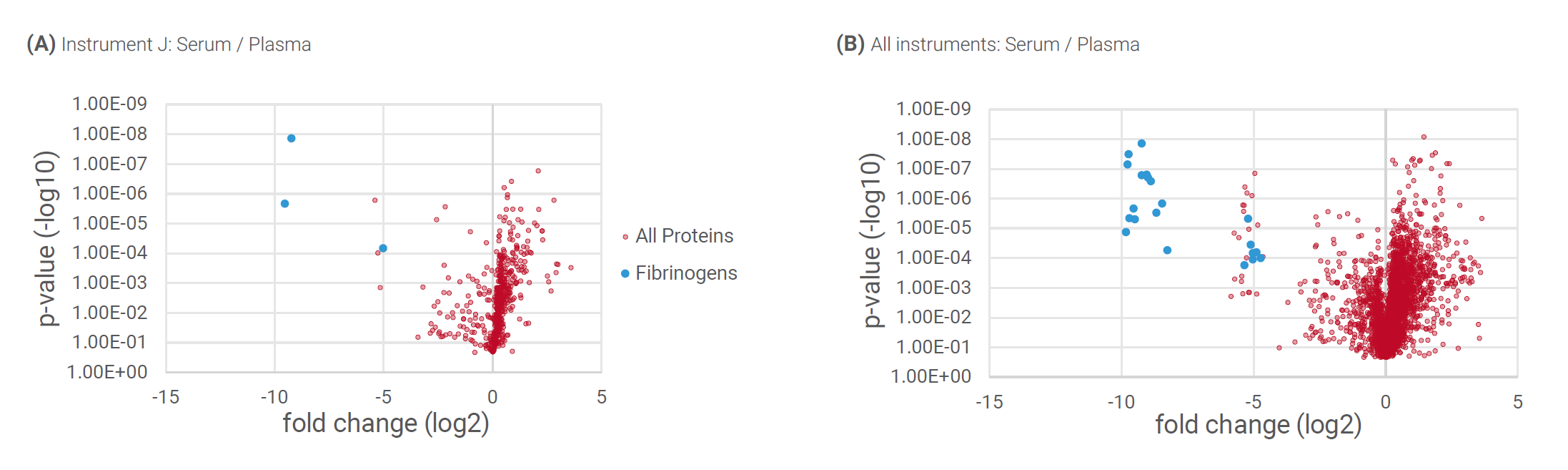
Figure 1: Analysis of plasma versus serum from the same blood draw. Fibrinogens, clotting factors, and certain calcium binding proteins were detected as significantly different, both on a single instrument (A) and multiple instruments (B).
Exceptional Analytical Depth
Analysis of plasma and serum pools showed excellent analytical depth with an average of 480 proteins (695 peptides) in plasma (Figure 2A) and serum (Figure 2B) across more than six orders of magnitude in dynamic range. The low coefficients of variation per condition proved great quantitative accuracy, while the low total variance across acquisition systems indicated minor instrument-specific effects.
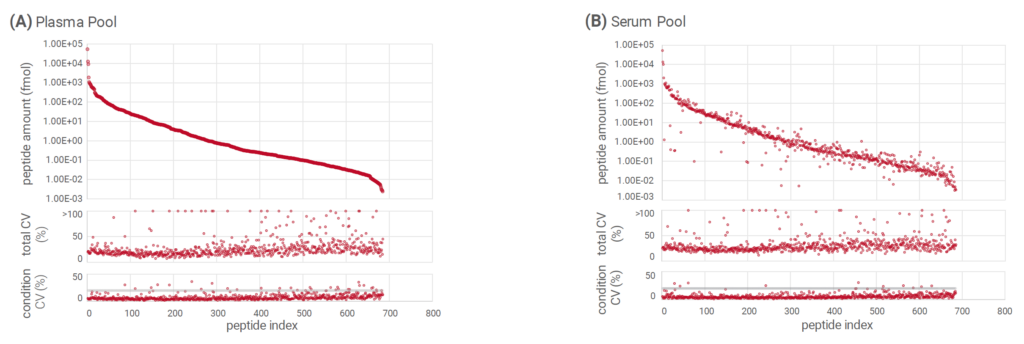
Figure 2: In plasma (A) and serum pools (B), an average of 480 proteins (695 peptides) were quantified across more than six orders of magnitude.
Consistent Biological Results Across Acquisition Systems
The prostate-specific antigen (PSA), is an important biomarker that increases in abundance in prostate cancer patients. PSA was consistently quantified in the plasma of a patient in all LC-MS systems (Figure 3A). Absolute amounts of PSA were measured with high accuracy in both serum (Figure 3B) and plasma samples (Figure 3C), while a higher fluctuation was observed in label-free intensities.
Although PSA increases in prostate cancer patients, overall, it is a very low abundant protein, and its detectability can be influenced by instrument setup (Figure 3D-E). Our workflow shows that different acquisition systems can effectively detect the difference in abundance of PSA between prostate cancer patients and healthy individuals.
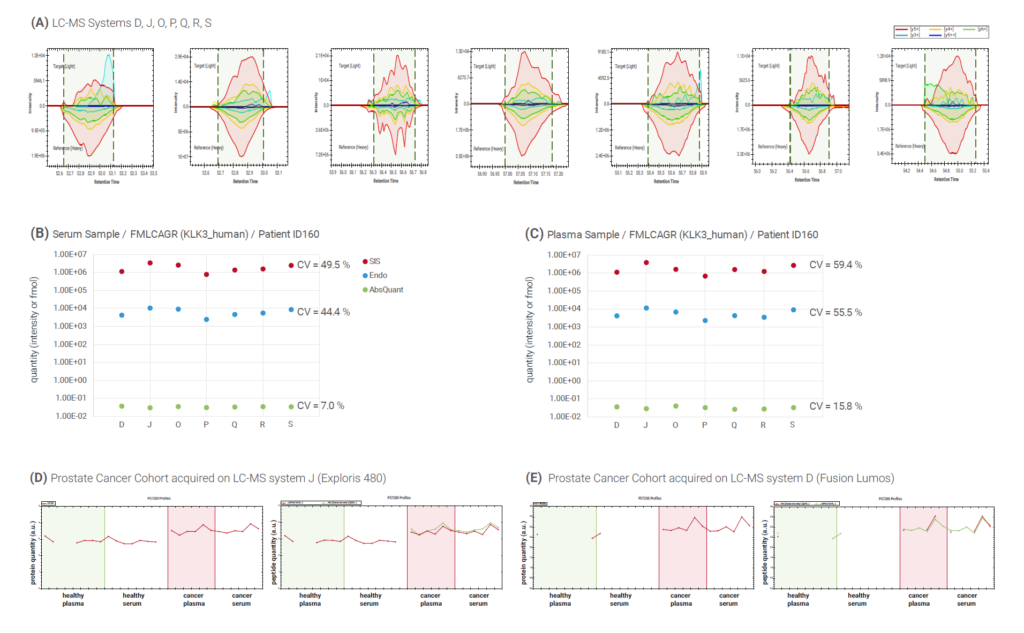
Figure 3: Prostate-specific antigen (PSA) was quantified consistently in a cancer patient’s plasma across seven LC-MS systems (A). Absolute amounts of PSA were measured with a CV <20% in serum (B) and plasma (C). Detectability can be influenced by instrument setup. Targeted acquisition on the Exploris480 platform (D) detected more PSA peptides more consistently than the same method on the Fusion Lumos platform (E).
Although PSA increases in prostate cancer patients, overall, it is a very low abundant protein, and its detectability can be influenced by instrument setup (Figure 3D-E). Our workflow shows that different acquisition systems can effectively detect the difference in abundance of PSA between prostate cancer patients and healthy individuals.
PQ500™: An Important Tool for Large-scale Analyses
Overall, the consistent results between different LC-MS systems confirm that our PQ500™ Reference Peptide kit in combination with targeted proteomics is a powerful tool for large-scale plasma and serum analyses that are spread across multiple analytical platforms.
We found that instrumentation only marginally influenced quantitative results and the statistical outcome, while the sensitivity of the analysis was mostly dependent on the MS set-up and performance state.
Workflows using our PQ500™ Reference Peptides kit seamlessly integrate with Biognosys’ SpectroDive™ and Spectronaut™ software. To find out more don’t hesitate to contact us at or visit the PQ500™ Reference Peptides kit page here.
This article was first published in The French Proteomics Society’s Newsletter.

