Immunotherapy is a powerful new paradigm in cancer treatment, manipulating the immune system to help the body attack cancer cells. Although immunotherapy has generated survival benefits in several cancers [1, 2], challenges remain to achieve the full promise of the therapy.
Immunotherapy does not work for all cancer patients – currently, only 15-20% of patients achieve lasting results from immunotherapy [3] – and there is a need for more personalized types of treatment to shift this statistic.
Using insights from individual biology, clinicians could better match patients to therapies and understand when a patient may need to switch treatments. Furthermore, having effective biomarkers to predict response or resistance could help to maximize the benefits of immunotherapy.
Next-generation proteomics is a powerful way of achieving these goals. It can go deep into the proteome to discover proteins missed by other approaches and reveal novel indicators of disease progression or treatment response.
Recent findings from the PRINCE trial [4], in collaboration with the Parker Institute for Cancer Immunotherapy (PICI) in San Francisco, are a powerful demonstration of this technology in action.
The PRINCE Trial
The PRINCE trial, a phase II clinical trial conducted by PICI, set out to determine the efficacy of combination immunotherapy and chemotherapy treatment for pancreatic cancer.
Pancreatic cancer is notoriously difficult to treat, with an average five-year survival rate of just 11% [5]. The disease often demonstrates resistance to common forms of immunotherapy, including checkpoint inhibitors, due to the robust inflammatory tumor microenvironment [6].
PRINCE assessed the effects of treatment with two immunotherapy drugs – nivolumab, a PD-1 inhibitor, and sotigalimab, a CD40 agonist – delivered in combination with the standard chemotherapy agent Gemcitabine/nab-Paclitaxel (GnP).
The PICI team wanted to know which form of treatment was most effective. Moreover, they wanted to find biomarkers to help track this efficacy and understand which patients would be most suitable for which treatment.
To answer these questions, as part of our Grant Program, we performed unbiased mass spectrometry-driven proteomics with our next-generation platform TrueDiscovery™️ in blood serum from patients enrolled in the trial. This allowed us to analyze samples at regular intervals and assess how patients were responding to therapy over time.
We analyzed 211 samples and quantified more than 2,600 proteins. Our deep analysis can achieve a 17-fold enrichment of low abundance proteins, many of which have biological and disease relevance.
Our approach also offers peptide-level resolution, which means we can look at protein sub-domains and PTMs. In this study, we identified several peptides from cancer-relevant proteins, including CD33 and EGFR.
Importantly, this approach dramatically increases the depth of analysis. More than half of the identified proteins would not have been picked up by traditional proteomics approaches because they were in the low abundance region of plasma (Figure 1).
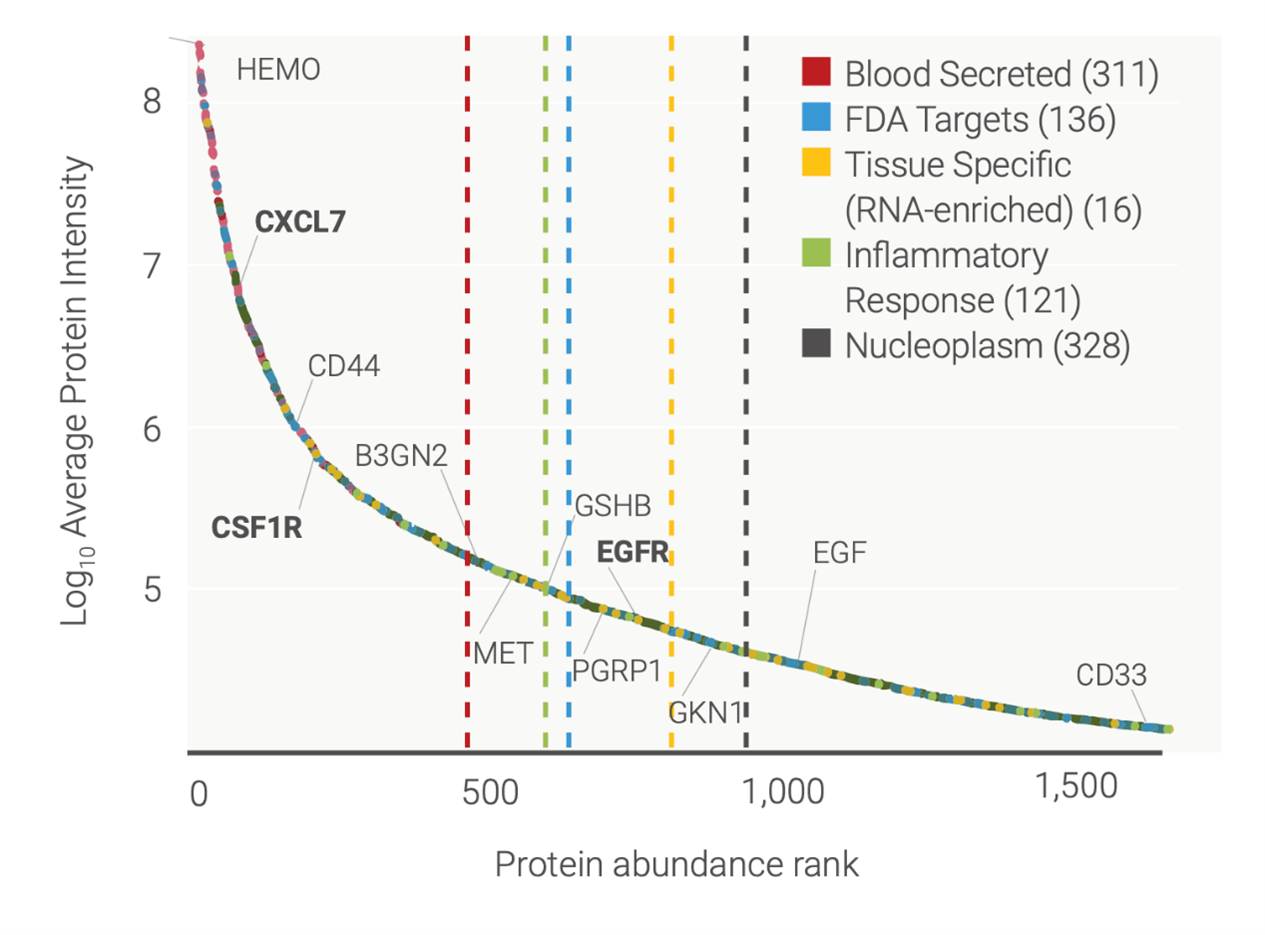
Figure 1: Range of proteins detected by TrueDiscovery™ in serum during the PRINCE trial, with low-abundant proteins shown on the right-hand side of the graph (protein abundance rank around 500 and above).
Treatment-specific Biomarkers
Thanks to our proprietary machine learning analysis, we were able to define several biomarker candidates that could distinguish different treatment arms and timepoints (Figure 2A).
Patients treated with nivolumab plus chemotherapy showed increases in proteins involved in T cell activation and immune cell migration, while those treated with sotigalimab plus chemotherapy had an increase in proteins associated with the activation of helper T cells, innate immunity, and chemokine signaling (Figure 2B, 2C) [7].
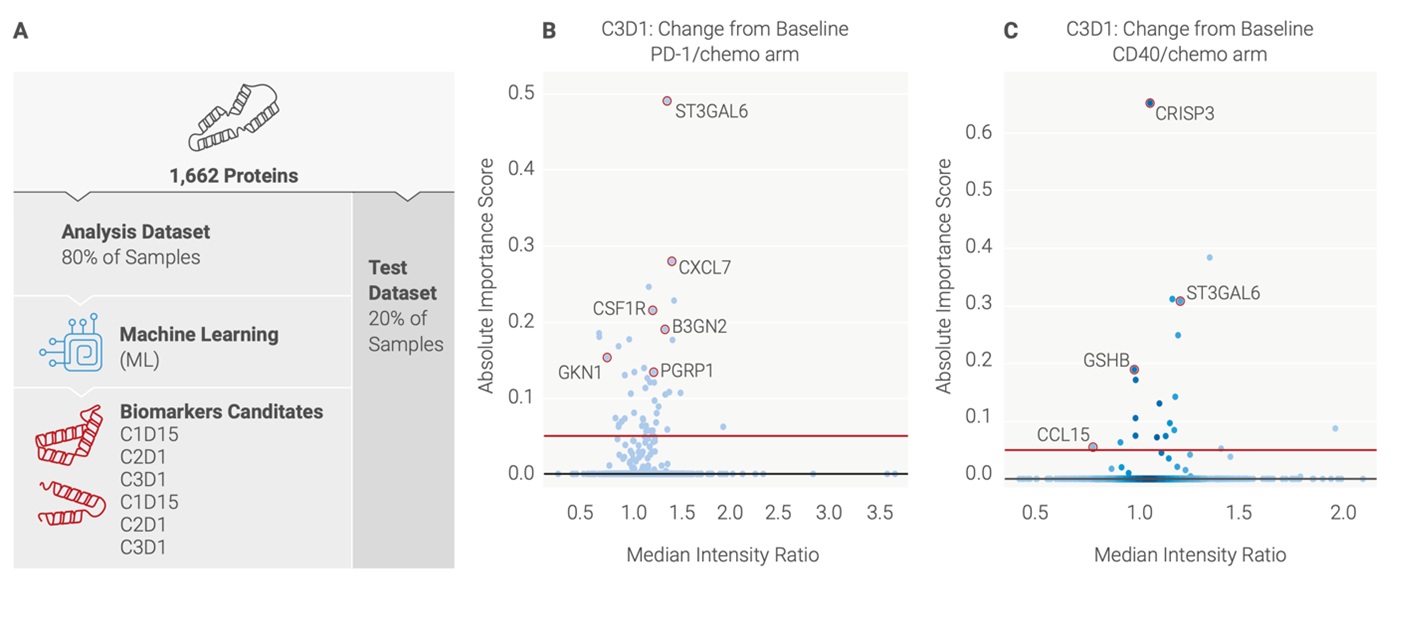
Figure 2: A) Overview of data processing pipeline (B) Altered proteins in PD-1/chemo arm of treatment (C) Altered proteins in CD40/chemo arm of treatment.
The insights from proteomics therefore showed that these two different treatment approaches generate unique immune responses, which are consistent with their modes of action. We also identified proteins that were not picked up by a targeted proteomics approach, several of which were novel biomarker candidates (Figure 3).
These insights could be used to better understand how immunotherapies work and help to deliver the right combination of treatments to the right patients, maximizing the chance of a good clinical outcome. They can also be critical during early phase clinical studies, helping to inform key development decisions and to stratify patient cohorts more effectively.
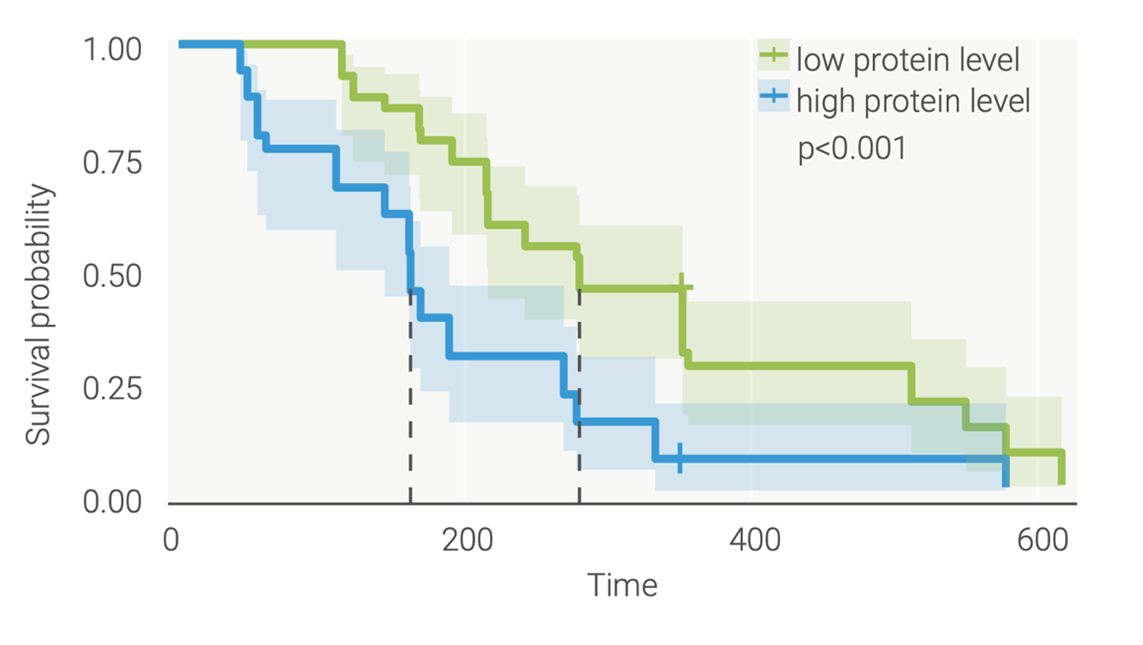
Figure 3: TrueDiscovery™ enabled the identification of novel biomarker candidates that have the potential to differentiate responders from non-responders. Several biomarkers were found to be predictive of clinical outcomes (overall survival) at baseline.
Watch this webinar featuring our principal scientist Marco Tognetti, Ph.D. and our collaborators from the PICI institute Deena Maurer, Ph.D., and Diane Da Silva, Ph.D., to find out more about our role in the PRINCE trial and the insights that proteomics can provide for immunotherapy research.
The Power of Proteomics for Immuno-oncology Research
Proteomics offers many advantages for immuno-oncology research in addition to biomarker discovery. One further example is the field of immunopeptidomics – mapping the many thousands of peptides presented on the surface of cancer cells, which play a key role in the immune response.
Mass spectrometry proteomics is the only technology that can extensively characterize the wealth of immunopeptides and help to identify new therapeutic targets for cancer, including candidates for vaccines and cell therapies.
At Biognosys, we offer integrated proteomics solutions across the whole drug discovery and development pipeline, powered by our next-generation platforms TrueDiscovery™, TrueTarget™ and TrueSignature™. Our services range from fundamental discovery science such as immune cell profiling to biomarker detection in biofluids or tissue, developing novel biomarker panels for pre-clinical or clinical research, immunopeptidome profiling, and much more.
Get in touch with our expert team to learn how our proteomics services can accelerate your immuno-oncology research.

